Nine pharmaceutical companies were publicly punished.
Many pharmaceutical companies were fined for producing and selling inferior drugs! Recently, Shanghai Food and Drug Administration issued an administrative penalty decision.  The contents of the announcement show that the "Dissolution" of omeprazole enteric-coated capsules (specification: 20mg, batch number: 201211) produced and sold by Shanghai You Mei Pharmaceutical Co., Ltd. did not meet the requirements after supervision and inspection. It is reported that in December 2020, the parties produced 23,970 boxes of omeprazole enteric-coated capsules, and all of them were sold out. From September 29 to December 24, 2021, the parties took the initiative to recall the products, recalling a total of 6085 boxes. The parties actually sold 17,885 boxes of this batch of drugs. The value of this batch of drugs is RMB 83,895, and the illegal income of the parties is RMB 55,396.02. [Result] According to Article 117 of the Drug Administration Law, Shanghai Food and Drug Administration imposed the following administrative penalties on Shanghai You Mei Pharmaceutical Co., Ltd.: confiscation of 6085 illegal omeprazole enteric-coated capsules (specification: 20mg, batch number: 201211); Confiscation of illegal income of RMB 55,396.02; A fine of one million five hundred thousand yuan only (1,500,000 yuan). It is understood that omeprazole enteric-coated capsules, as a commonly used medicine for the treatment of stomach diseases, occupy a high market share in digestive diseases. According to the data in the Minenet, the overall market size of omeprazole enteric-coated capsules in China domestic market is about 2.278 billion yuan in 2020. However, the omeprazole enteric-coated capsules produced by Shanghai You Mei Pharmaceutical Co., Ltd. were found to be unqualified and punished, which may have a certain impact on the company. Therefore, it is hoped that the enterprise will strengthen the supervision of drug production.If you commit another crime next time, the punishment may be more severe.
The contents of the announcement show that the "Dissolution" of omeprazole enteric-coated capsules (specification: 20mg, batch number: 201211) produced and sold by Shanghai You Mei Pharmaceutical Co., Ltd. did not meet the requirements after supervision and inspection. It is reported that in December 2020, the parties produced 23,970 boxes of omeprazole enteric-coated capsules, and all of them were sold out. From September 29 to December 24, 2021, the parties took the initiative to recall the products, recalling a total of 6085 boxes. The parties actually sold 17,885 boxes of this batch of drugs. The value of this batch of drugs is RMB 83,895, and the illegal income of the parties is RMB 55,396.02. [Result] According to Article 117 of the Drug Administration Law, Shanghai Food and Drug Administration imposed the following administrative penalties on Shanghai You Mei Pharmaceutical Co., Ltd.: confiscation of 6085 illegal omeprazole enteric-coated capsules (specification: 20mg, batch number: 201211); Confiscation of illegal income of RMB 55,396.02; A fine of one million five hundred thousand yuan only (1,500,000 yuan). It is understood that omeprazole enteric-coated capsules, as a commonly used medicine for the treatment of stomach diseases, occupy a high market share in digestive diseases. According to the data in the Minenet, the overall market size of omeprazole enteric-coated capsules in China domestic market is about 2.278 billion yuan in 2020. However, the omeprazole enteric-coated capsules produced by Shanghai You Mei Pharmaceutical Co., Ltd. were found to be unqualified and punished, which may have a certain impact on the company. Therefore, it is hoped that the enterprise will strengthen the supervision of drug production.If you commit another crime next time, the punishment may be more severe.
A pharmaceutical company was fined again for producing inferior drugs. In addition, in recent days, Fujian Food and Drug Administration has also issued an administrative punishment information disclosure form, which has publicly punished two pharmaceutical companies.  It was found that the companies punished this time were Fujian Qiancao Pharmaceutical Co., Ltd. and Fujian Yangzu Pharmaceutical Co., Ltd. Among them, Fujian Yangzu Pharmaceutical Co., Ltd. was ordered by the Food and Drug Administration to immediately correct and give a warning for failing to implement the drug traceability system in accordance with the regulations. Fujian Qiancao Pharmaceutical Co., Ltd., however, was given a warning by the Food and Drug Administration, confiscated illegal income and fined 100,000 yuan, with a total penalty of 101,700 yuan, because the traditional Chinese medicine decoction piece Gentiana macrophylla produced and sold was found to be unqualified. It is worth noting that this pharmaceutical company has been punished four times by the Food and Drug Administration in the past two years.
It was found that the companies punished this time were Fujian Qiancao Pharmaceutical Co., Ltd. and Fujian Yangzu Pharmaceutical Co., Ltd. Among them, Fujian Yangzu Pharmaceutical Co., Ltd. was ordered by the Food and Drug Administration to immediately correct and give a warning for failing to implement the drug traceability system in accordance with the regulations. Fujian Qiancao Pharmaceutical Co., Ltd., however, was given a warning by the Food and Drug Administration, confiscated illegal income and fined 100,000 yuan, with a total penalty of 101,700 yuan, because the traditional Chinese medicine decoction piece Gentiana macrophylla produced and sold was found to be unqualified. It is worth noting that this pharmaceutical company has been punished four times by the Food and Drug Administration in the past two years.
On December 1, 2021, an administrative penalty information form issued by Fujian Province showed that the Chinese medicine decoction piece wine dogwood meat (batch number is 200501, specifications are unified, and packaging specifications are 1kg/bag) produced by the pharmaceutical company failed to meet the requirements after inspection. On September 8, 2020, the parties concerned sold all the above wine dogwood meat and got a sum of 2,760 yuan. Therefore, the Drug Administration Law of the Food and Drug Administration stipulates that the company was fined a total of 1,002,760 yuan.
On June 18, 2021, Fujian Food and Drug Administration issued a notice saying that the Cortex Dictamni Radicis, Radix Peucedani and roasted Herba Epimedii produced by Fujian Qiancao Pharmaceutical Co., Ltd. did not meet the requirements, and they were fined a total of 33,610.8 yuan.
In November 2020, Fujian Food and Drug Administration issued an announcement that the acanthopanax bark produced by this enterprise did not meet the project requirements, and it was fined 2,457 yuan according to law.
In October, 2020, Fujian Food and Drug Administration issued a penalty message. Because the produced Chinese herbal pieces of Cortex Lycii and Rhizoma Menispermi did not meet the requirements of the project, they were fined 2.5 times the value of 8604 yuan, totaling 21510 yuan.
In just over a year, the company fined more than one million yuan for producing inferior drugs, involving as many as eight unqualified varieties. Under such a high unqualified rate, the Food and Drug Administration will inevitably strengthen supervision over the company. It is worth mentioning that during November 2020, Fujian Food and Drug Administration issued a notice saying that Fujian Qiancao Pharmaceutical Co., Ltd. applied to cancel the Pharmaceutical Production License and Pharmaceutical GMP Certificate of the parties according to law, and then the company stopped production.
Multi-provincial Food and Drug Administration publicizes illegal activities of pharmaceutical companies Coincidentally, the local medical health station also noticed that at least six pharmaceutical companies have been punished by publicity in the past month, involving enterprises in Guangxi, Inner Mongolia, Hubei Province and other places. Among them, Baotou Yiren Pharmaceutical Co., Ltd., Bozhou Jinshaotang Chinese Herbal Pieces Co., Ltd. and Anhui Wei Wu Chinese Herbal Pieces Technology Co., Ltd. were punished by the Food and Drug Administration for producing and selling inferior drugs; Guangxi Jirentang Pharmaceutical Co., Ltd. and Shanghai Pharmaceutical Holdings Zhenjiang Co., Ltd. were punished for selling inferior drugs; Hubei Huquan Pharmaceutical Co., Ltd. was punished by Hubei Province for not strictly implementing the relevant requirements of good manufacturing practice. See the following table for details of specific penalties: 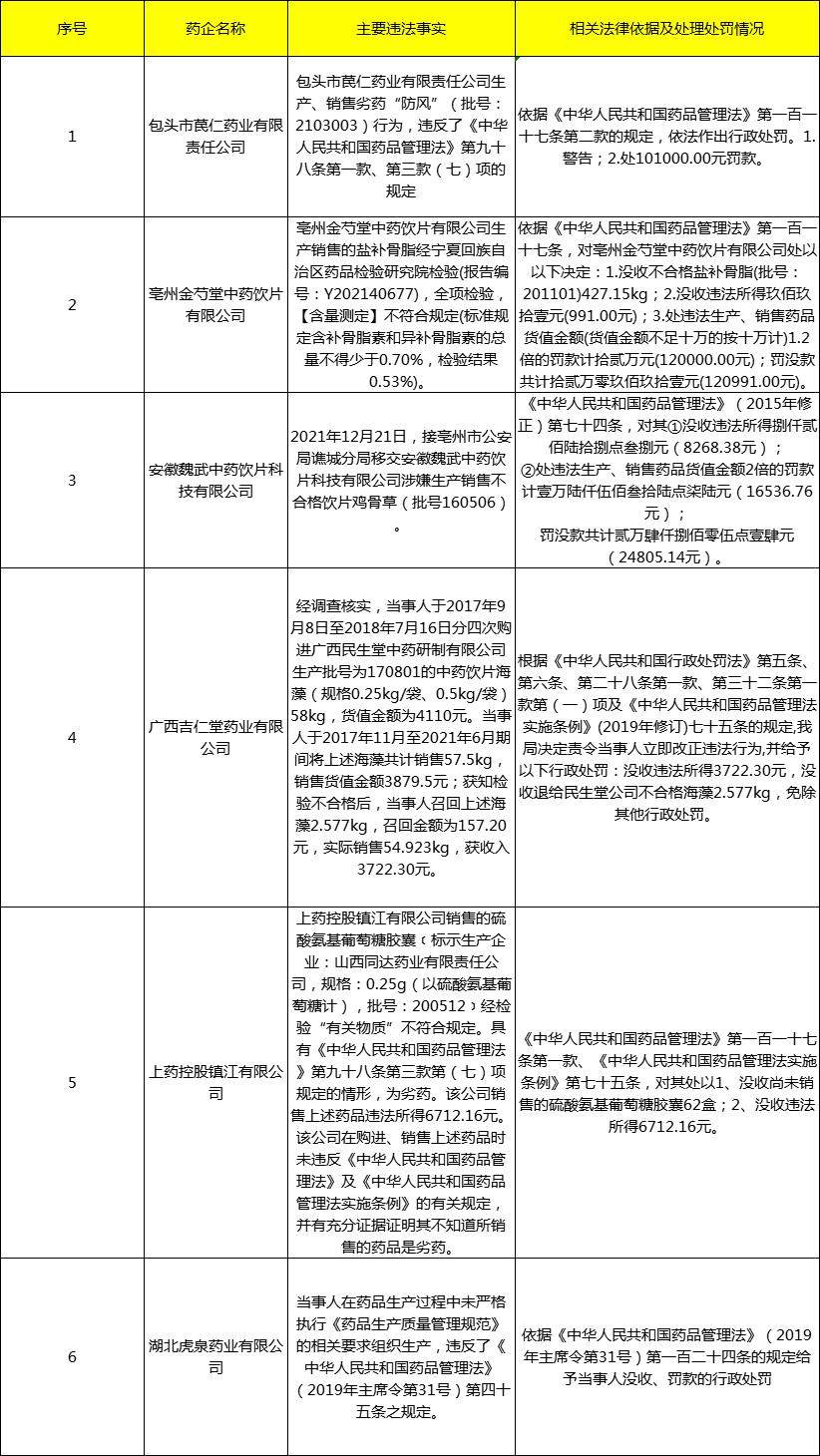
Under the strict supervision of the national pharmaceutical department, both pharmaceutical trading enterprises and production enterprises will be punished once they violate the provisions of the Drug Administration Law. Therefore, for enterprises, compliance and legal production and operation is the king of survival.
Source: Medical Health Local Station
Typesetting: Joyce
Editor: Adam